The Paragard litigations were one of a number of mass torts to make headlines in the final months of 2020. The first steps towards the formation of a multidistrict litigation (“MDL”) were taken in September 2020. The Paragard litigations were one of a number of mass torts to make headlines in the final months of 2020, with the first steps towards the formation of a multidistrict litigation (“MDL”) being taken in September 2020 and consolidation into an MDL occurring in December 2020.
What is Paragard?
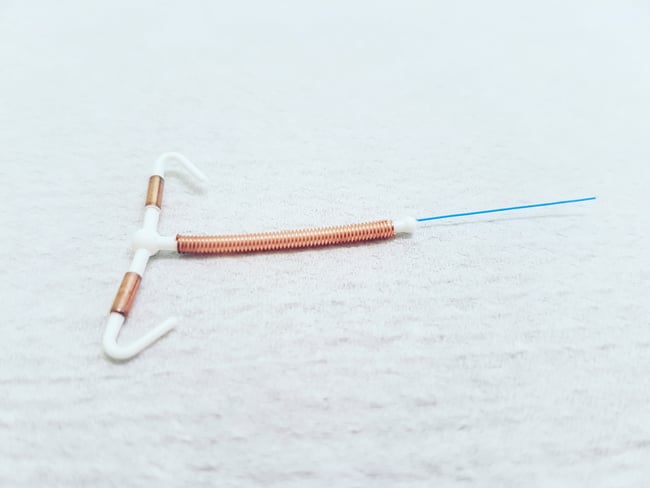
Paragard is a non-hormonal intrauterine device (“IUD”) that can provide long-term non-hormonal birth control for up to 10 years. The IUD is a T-shaped plastic frame made of polyethlene and barium sulfate that is inserted into the uterus. The copper wire that is coiled around the IUD produces an inflammatory reaction that is toxic to sperm and eggs. A monofilament polyethylene thread is tied through the tip of the IUD resulting in two white threads which then aid in the detection and removal of the IUD.
Paragard IUD was originally created by Duramed Pharmaceuticals in 1982 and came to market in 1984, at which time it was approved only for up to four years of continuous use. In 1994, the drug was approved for up to 10 years of continuous use. Paragard was purchased by Teva Pharmaceuticals USA Inc. (“Teva”) in 2009 and then subsequently sold in 2017 to Cooper Surgical Inc. (“Cooper”), which currently owns, sells and manufactures the product.
Both Teva and Cooper heavily marketed the drug as being safe, effective and having fewer side effects than other traditional birth control methods. However, Paragard IUD’s have a propensity to break at the arms upon explant resulting in serious injuries. In order to remove the IUD, patients are required to visit their healthcare provider. Physicians are instructed by the manufacturer to locate the two white threads and to pull gently until the IUD is expelled from the uterus. Ideally, the arms of the Paragard IUD are supposed to fold upward at the joint to aid in the removal, however, often the arms of the IUD break during removal. The resulting complications and injuries can potentially lead to surgery to remove the broken pieces of the device, which can cause infertility and pain.
Plaintiffs’ Allegations
Plaintiffs in Paragard litigations allege that defendant Teva’s marketing and promotional efforts overstated the benefits of the IUD and minimized and downplayed the risks associated with its use. The plaintiffs claim that while Paragard was marketed as one of the safest forms of birth control on the market, Teva withheld important safety information from healthcare providers and the public. Suits in the Paragard litigations similarly argue that defendant Teva knew, or should have known, that the drug was defective and unreasonably dangerous and that it could cause serious harm to individuals who used it due to the IUD’s tendency to break apart upon removal.
Plaintiffs pointed out that Teva and Cooper knew of the risks associated with the drug from their own trials, users’ complaints and independent third-party studies. Suits allege that since the defendant drug companies were aware, or reasonably should have been aware, of the dangerous issues surrounding the IUD, they purposefully and deliberately failed to adequately warn consumers, patients and medical providers of the increased risk of serious injury associated with using Paragard IUDs. In addition to the warnings that Paragard’s makers received directly from the FDA, since 2010, there have been over 1,600 reports of Paragard IUD breakage with over 700 of those reports classified as serious.
Procedural Status
Paragard litigations began to gain momentum in 2020 with plaintiffs petitioning the JPML to transfer and consolidate suits to an MDL centralized in the U.S. District Court in the Central District of California under Judge John A. Kronstadt. In December 2020, an attorney representing several plaintiffs in suits against Paragard and its manufacturer, Teva, urged the JPML to consolidate cases into an MDL in U.S. District Court in the Northern District of Georgia under Judge Leigh Martin May. Proponents of Judge May argue that the suits should be presided over by a woman due to the fact that the litigation centers around a women’s product. On December 16, 2020, the JPML issued a transfer order centralizing the suits into an MDL in the Northern District of Georgia under Judge May.




